The Benefits of Salicylic Acid
By Jeff Haag
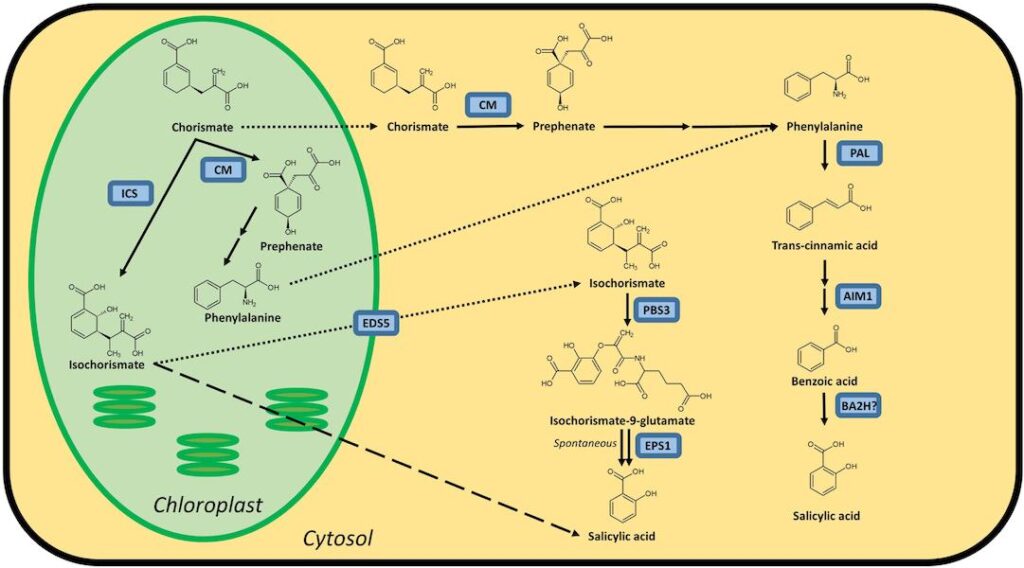
The ability of plants to develop acquired immunity after pathogen infection was first proposed in 1933. However, most of our knowledge about plant immune signaling was generated over the last three decades, following the discovery that salicylic acid (SA) is an endogenous defense signal. During this timeframe, researchers have identified two pathways through which SA can be synthesized, numerous proteins that regulate SA synthesis and metabolism, and some of the signaling components that function downstream of SA, including a large number of SA targets or receptors. In addition, it has become increasingly evident that SA does not signal immune responses by itself, but rather as part of an intricate network that involves many other plant hormones. Future efforts to develop a comprehensive understanding of SA-mediated immune signaling will therefore need to close knowledge gaps that exist within the SA pathway itself, as well as clarify how crosstalk among the different hormone signaling pathways leads to an immune response that is both robust and optimized for maximal efficacy, depending on the identity of the attacking pathogen.
SA is one of thousands of phenolic compounds, which consist of an aromatic ring bearing one or more hydroxyl substituents, that are synthesized by turf (Métraux and Raskin 1993). Traditionally, phenolics were assumed to be relatively unimportant, or even waste products, and, thus, were categorized as secondary metabolites (Raskin 1992). However, subsequent studies revealed that plant phenolics have many important functions. Some play critical roles in defense against abiotic stresses, whereas others are important cell-wall structural components, pigments, allelopathic compounds, or signals that influence plant-microbe interactions (Dempsey et al. 2011; Métraux and Raskin 1993; Raskin 1992). In addition, some phenolics play key roles in resisting pest or microbial attack, or both, by serving as constitutively produced antibiotic compounds called phytoanticipins (VanEtten et al. 1994), inducible antimicrobial compounds known as phytoalexins, or signals that activate antimicrobial defense responses (Pieterse et al. 2009; Raskin 1992; Seyfferth and Tsuda 2014; Vlot et al. 2009).
Efforts to identify the function of SA have revealed that it affects a wide range of plant processes. In addition to influencing tolerance to various abiotic stresses (chilling, heat, drought, heavy metal, UV radiation, salinity or osmotic stress) and inducing resistance to biotic (pathogen-associated) stress, exogenously supplied SA affects numerous aspects of plant growth and development, including seed germination, vegetative growth, root initiation and growth, photosynthesis, respiration, the alternative respiratory pathway, glycolysis, and the Krebs cycle (Hayat et al. 2010; Khan et al. 2015; Malamy and Klessig 1992; Miura and Tada 2014; Rivas-San Vicente and Plasencia 2011).
SA has been recognized as a regulatory signal mediating plant response to abiotic stresses such as drought (Munné-Bosch and Peñuelas, 2003; Chini et al., 2004), chilling (Janda et al., 1999; Kang and Saltveit 2002), heavy metal tolerance (Metwally et al., 2003; Yang et al., 2003; Freeman et al., 2005), heat (Larkindale and Knight, 2002; Larkindale et al., 2005), and osmotic stress (Borsani et al., 2001), and there are several products currently on the market for sports field managers at the present time. However, most of the research on this hormone has focused on its role in the local and systemic response against microbial pathogens, and on defining the transduction pathway leading to gene expression induced by SA. Again, there are several reviews on this subject (Klessig and Malamy, 1994; Durner et al., 1997; Shah, 2003; Durrant and Dong, 2004; Vlot et al., 2009). Plants perceive pathogens such as viruses, bacteria, fungi and oomycetes or abiotic stress, and respond by a characteristic innate immune response that leads to the induction of local and systemic resistance. It concomitantly leads to the production of SA, an innate immune signal responsible for transcriptional changes that result in resistance in the infected and neighboring cells (Vlot et al., 2009).
Recent evidence also suggests that SA is an important regulator of photosynthesis because it affects leaf and chloroplast structure (Uzunova and Popova, 2000), stomatal closure (Mateo et al., 2004; Melotto et al., 2006), chlorophyll and carotenoid contents (Rao et al., 1997; Chandra and Bhatt, 1998; Fariduddin et al., 2003), and the activity of enzymes such as RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) and carbonic anhydrase (Pancheva and Popova, 1998; Slaymaker et al., 2002).
Again, it has been observed that the effects of exogenous SA on photosynthesis parameters differ depending on the dose and plant species tested. High SA concentrations (1–5 mM) cause a reduction in the photosynthetic rate (PN) and RuBisCO activity in barley plants (Pancheva et al., 1996), and reduced chlorophyll contents in cowpea, wheat, and Arabidopsis (Rao et al., 1997; Chandra and Bhatt, 1998; Moharekar et al., 2003). The decline of RuBisCO activity was attributed to a 50% reduction in protein levels compared with non-treated plants (Pancheva and Popova, 1998), while total soluble protein decreased ∼68%. Exogenous SA induces alterations in leaf anatomy that consist of a reduced width of the adaxial and abaxial epidermis, and of the mesophyll tissue. Such changes correlate ultrastructurally with an increase in chloroplast volume, swelling of grana thylakoids, and coagulation of the stroma (Uzunova and Popova, 2000). Thus, the diminished photosynthetic activity at high concentrations of SA is due to its effects on the thylakoid membranes and light-induced reactions linked to them.
Stomatal closure is another important factor for photosynthesis, and is subjected to control by various phytohormones (reviewed by Acharya and Assmann, 2009). Recent evidence links stomatal closure to innate plant immunity, highlighting the role of SA in the function of the guard cells (Melotto et al., 2006). In Arabidopsis, 0.4 mM SA induces rapid stomatal closure within 2 h and a 4-fold reduction of stomatal gas exchange (Mateo et al., 2004). Endogenous SA levels promote stomatal closure upon pathogen attack.
SA is a defense hormone which has its role in both local resistances as well as in systemic acquired resistance in plants (Zhang et al. 2010). Delaney et al. (1994) showed that salicylic acid accumulation is essential for expression of multiple modes of plant disease resistance. They found that turfgrass cannot accumulate SA because of the expression of bacterial enzyme salicylate hydroxylase (a flavoprotein monooxygenase that catalyzes the conversion of salicylate to catechol according to reaction: salicylate + NADH + 2H+ + O2 ⇌ Catechol + NAD+ + H2O + CO2). In addition to making these plants unable to induce systemic acquired resistance, this defect also increased their susceptibility to viral, fungal and bacterial pathogens. Even host–pathogen combinations which are otherwise part of genetic resistance were also affected in this defect (Delaney et al. 1994; Anand et al. 2008).
Exogenous application of salicylic acid or its functional analogs (like 2,6-dichloroisonicotonic acid and benzo-(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester) induces systemic acquired resistance (SAR) in plants, there by providing a considerable protection against various biotic stress like resistance to pathogens (Achuo et al. 2004; Wang et al. 2005; Hayat et al. 2010). Depending on its involvement in local immunity, systemic acquired resistance, and its participation in both biotic and abiotic plant stress responses, salicylic acid is reported to have many therapeutic actions against several diseases and toxicities in plants in addition to its involvement in plant growth and development. Exogenous application of salicylic acid induces the expression of pathogenesis-related genes and also encourages resistance against various pathogens of viral, bacterial, oomycete and fungal origin in a variety of plants (Ryals et al. 1996; Shah and Klessig 1999; Pasquer et al. 2005; Makandar et al. 2006). Salicylic acid activates a group of events resulting in the inhibition of viral replication and their cell-to-cell and long-distance transmission in plants (Singh et al. 2011).
Relationship of SA with antioxidant system and its impact on the plants exposed to stress
Stressful environments induce the generation of reactive oxygen species (ROS) such as superoxide radicals (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (OH−) etc. in plants thereby creating a state of oxidative stress in them (Elstner, 1982, Haag, 2008, Halliwell and Gutteridge, 1988, Asada, 1994, Gille and Singler, 1995, Monk et al., 1989, Prasad et al., 1999, Panda et al., 2003a, Panda et al., 2003b). This increased ROS level in plants cause oxidative damage to biomolecules such as lipids, proteins and nucleic acids, thus altering the redox homeostasis (Smirnoff, 1993, Gille and Singler, 1995). When applied exogenously at suitable concentrations, SA was found to enhance the efficiency of antioxidant system in turf plants. As sports field managers, many of us also use seaweed extracts to increase the antioxidant system to combat Reactive Oxygen Species.
Benefits to cool-season turfgrasses
Heat stress is a major factor limiting growth of cool‐season grasses in the warm climatic regions. Heat injury in cool‐season turfgrasses has been associated with oxidative stress (Liu and Huang, 2000). Increases in the production of AOS, such as O2− and H2O2, are typical plant responses to biotic and abiotic stress (Foyer et al., 1994, 1997). Excessive accumulations of AOS are potentially damaging to plant cells unless effectively detoxified by an antioxidant system (Foyer et al., 1994). Prevention of oxidative damage to cells during stress has been suggested as one of the mechanisms of stress tolerance (Kraus and Fletcher, 1994), which is attributed to enhanced antioxidant enzyme activity (Senaratna et al., 1988). Superoxide dismutase catalyzes the dismutation of the O2− into H2O2 and O2 (Elstner and Heupel, 1976). Catalase breaks down H2O2 Superoxide dismutase and CAT are the most effective antioxidant enzymes in scavenging AOS (Bowler et al., 1992). Kentucky bluegrass is the most widely used cool‐season turfgrass in temperate climates. Turf quality of Kentucky bluegrass often declines during summer.
Results have demonstrated that application of the 0.25 mmol SA solution was the most effective concentration for improving heat tolerance in Kentucky bluegrass plants. Previous studies with other cool‐season turfgrasses found that 0.5 mmol SA significantly increased heat tolerance in tall fescue seedlings (He et al., 2002), and 0.01 mmol SA was effective in increasing turf quality, leaf net photosynthetic rate, and suppressing lipid peroxidation in creeping bentgrass (Larkindale and Huang, 2004).
Jeff Haag is turf specialist at Xavier University, Cincinnati, Ohio. In the past he has served as sports turf manager/golf course superintendent at Bowling Green State University, assistant sports turf manager at the University of Louisville, sports turf specialist at John Carroll University, and athletic grounds manager at Nova Southeastern University.
Editor’s Note: This article originally appeared in the December 2020 issue of SportsField Management, sister publication to Landscape Business.


