The Relationship Between Water and Soils

Water management has become a major issue in the sports turf industry over the last decade. In the past, water was considered an unlimited resource and was a concern only when an occasional drought occurred. Today, all regions have experienced a change in attitude regarding water. Influenced by rising costs, recurrent drought, use restrictions, politics and social pressures, turf managers are expected to do more with less.
First, the cost of water in many markets, including “water-rich” regions, has increased dramatically since the turn of the century. Surveys have revealed that the cost of water has risen by 25% to 30% in many municipalities, with increases reaching as high as 300% or more in some regions. Is money better spent on other budgetary items than on irrigation? Whether working on the professional level or with a local youth complex, finding ways to manage water from an economical sense has become important to everyone.
Second, the recent severe droughts in the western US, especially California and Texas, have focused property managers and policy makers to reconsider how to best manage water resources. These droughts have reduced some water reservoirs to less than 25% of capacity and have stressed ground water supplies. We have read widely of wells going dry during these times, and many areas have imposed turf and landscape watering restrictions.
Consumers have different perspectives about water when it becomes scarce. There are different definitions of drought depending on who is defining it. These include:
- Meteorological. A measure of departure of precipitation from normal. This is due to climatic differences. What might be considered a drought in one location of the country may not be a drought in another location.
- Agricultural. Refers to a situation where the amount of moisture in the soil no longer meets the needs of a particular crop, including turf and ornamentals.
- Hydrological. This occurs when surface and subsurface water supplies are below normal.
- Socioeconomic. This refers to the situation that occurs when physical water shortages begin to affect people.
One can imagine how conflicts can occur between various water consumers based on the drought definition that applies to them. Each consumer group believes its water requirements should be primary. Governments must consider the desires or various clientele and may struggle to find balance when developing water policy. In many cases, the sports turf industry becomes a political target, particularly with regards to golf. Often regarded as a “sport for the rich,” water use on the courses takes heat as a wasteful luxury. With the assumption that green grass is the result of excess water use, highly maintained athletic complexes, parks and other sports fields may experience some of the same social pressures.
In the turfgrass industry, we are most concerned with the agricultural definition of drought. Inadequate soil moisture can occur at any time, even in “water-rich” regions. Agricultural drought can be caused by soil conditions, cultural practices or a number of other factors. The challenge is managing moisture throughout the soil profile so it can be made available to the plant. This article will focus on technologies that help managers use the water they can afford or are allotted by the most efficient means.
Beyond the advancements in irrigation hardware and software, there are many other technologies available for the management of water in the soil. They can have very different modes of activity, and each has been designed for a specific function and purpose. Understanding each technology and soil/water interaction will help turf managers decided which strategy is best suited for their specific situation. The first step in deciding what technology to use is to diagnose the reason for a water problem.
Three fates of water
With each irrigation cycle or rainfall, water will succumb to one of three inevitable fates. The desired outcome results in water entering the soil system, being taken up by the plant, and eventually lost to transpiration. Unfortunately, the forces of gravity and evaporation are constantly working to move water away from plant roots. Gravity plays a role in water lost to runoff, channeling and percolation. When it comes to evaporation, most people consider the evaporative loss that occurs immediately, before water has entered the soil; however, as soils dry between watering, the bonds that hold water molecules together break, causing liquid water to dissipate into its gaseous form. The resulting water vapor is not usable by plant roots and is continually lost to the atmosphere above. Additional irrigation to compensate for water lost to gravity or evaporation may be ineffective based on a number of factors, including soil chemical and physical properties, which affect infiltration and soil holding capacity.
Failure for water to move into or infiltrate the soil. Water may sit on top of a turf or soil surface or flow across that surface away from the intended target. It may find a site to drain through soil, but not disperse evenly into and throughout the rootzone. There are a number of causes for this, but let’s consider three possibilities. The first would occur with soils or irrigation water containing high levels of sodium salts. Sodium breaks down soil structure by removing the ability of soil particles to be “glued” together to construct various formations. Soil structure allows for the rapid movement of water into and throughout the soil. Salts may also physically “seal” the soil, preventing water penetration. The solutions for this are either calcium or acid. Calcium containing products, such as gypsum, liquid calcium chloride or calcium nitrate, etc., displace sodium ions and allow for the restoration of soil structure. Sulfuric acid, reacted with urea for safety, will convert lime that occurs naturally or is applied to soil, into gypsum, which supplies the calcium needed to make high salt conditions more permeable.
The second cause may be bicarbonates. Bicarbonates are common in regions where irrigation water comes from limestone aquifers. The bicarbonates will build up in a similar manner as salts and cause a “sealing” of the soil. A simple test for this condition is to drip some vinegar (an acid) solution on the questionable area. If the drop causes a fizz, then high bicarbonates are most likely present. These conditions may be treated with acids or acid forming materials that break down the bicarbonates into CO2 and water. These treatments would include acid materials injected through an irrigation system or applications of acid forming fertilizers, such as ammonium sulfate or ammonium thiosulfate liquid.
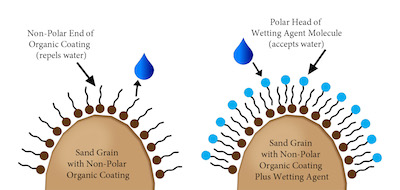
A third possibility may be caused by a waxy, organic coating on soil particles. The waxy coating comes from the decay of organic materials and certain species of fungi that exude waxy substances. These waxy coatings form non-polar hydrophobic surfaces that have no charge to attract water molecules. The water does not enter and disburse through the soil. The only polar surfaces are the surfaces of other water molecules. The water will find the path of least resistance and may either flow across the surface and/or find a channel through the soil profile to drain, thanks to gravity. The most common solution to this situation is the use of a penetrant, such as a surfactant or wetting agent.
Water channeling through soils. Beyond the influence of salts, bicarbonates or hydrophobic conditions, there may be other times when water will not disperse evenly through the soil. This may occur as a result of varying soil structure, compaction, inconsistent composition, layering, etc. Water will always following the path of least resistance and may drain through channels in the profile, leaving adjacent areas void of adequate water to supply plants. As a result, these areas become much more susceptible to drought or wilt, even under ideal irrigation coverage. These conditions are a primary cause of localized dry spots (LDS). The use of wetting agents or surfactants combined with cultural practices including aeration is often used to alleviate water loss to channeling.
Total water holding capacity of soil. Soil has the ability to hold a certain amount of water. The maximum amount of water soil is able to hold is generally referred to as “field capacity.” Field capacity is the condition that occurs after soil is completely saturated and excess water is allowed to drain away. The remaining water is either used by plants or lost to evaporation until the soil reaches the “permenant wilting point.” The permenant wilting point occurs when soil moisture drops so low that plants wilt and cannot recover. The amount of water available to the plant between field capacity and the permenant wilting point is referred to as the “total available water holding capacity” of the soil. This can be affected by soil texture, organic matter content, the ability of the soil to accept water as well as other factors.
Heavy textured soils with higher percentages of clay and silt will hold more water than lighter soils with high percentages of sand. Soils containing higher levels of organic matter will hold greater quantities of water than soils low in organic matter. Soils with a high level of organic non-polar coatings on soil particles will resist the acceptance of water and will hold less water than soils with less non-polar coatings. Sandy soils are more suseptible to non-polar coatings.
Products that help soil retain greater quantities of water and for longer periods of time include hygroscopic humectants, polymers, and surfactants/wetting agents.
Technologies for optimizing soil moisture management
Hygroscopic humectants. Though they are not new to the industry, hygroscopic humectants are continuing to gain notoriety with sports turf professionals as products that are very effective at reducing overall water requirements. With a history in golf, these products have been gaining greater attention due to their recent performance in drought stricken areas of California, Texas and other western states.
Hygroscopic humectants manage and conserve water through two modes. As the name suggest there is a hygroscopic compontent and a humectant component. Each has a critical function in the performance of the technology. The mode of action of the hygroscopic component is to condense soil water vapor or soil humidity back into liquid droplets of water. The hygroscopic ability of these materials can be compared to condensation or “sweat” that occurs on the side of a cold drink. Rootzone humidity that cannot be absorbed by plant roots is converted into plant usable micro-droplets of water. There are other materials used in this industry that are hygroscopic in nature. These include fertilizers such as urea. The hygroscopic nature of some fertilizers may cause bridging in a spreader, or turns a bag of granular fertilizer into a “brick.” The same hygroscopic materials used in hygroscopic humectants are also used in certain foods, such as breads, and in toiletry groups (e.g., toothpaste) to keep them moist and pliable.
The humectant components hold the water droplets condensed by the hygroscopic components. Do not confuse a humectant with a humate. They are completely different substances with different molecular structures. The humectant component holds the droplets tightly enough to prevent it from leaving the proximity of the root, but lightly enough to allow the root to absorb the water through osmosis. The humectants in hygroscopic humectants are also used in cosmetics, shampoos, and other body care products where they help hold moisture in the skin and hair.
Available in both liquid and granular options, hygroscopic humectant technologies must be watered-in, at which point the active ingredients will coat plant roots, soil particles and organic particles in the rootzone. The hygroscopic humectant molecules are too large to be absorbed by the roots. Once these components attach to the roots and soil particles, they remain attached and are resistant to further movement in the soil. The ingredients are primarily derived from plant byproducts (some brands are rated at 93% biobased by the USDA BioPreferred Program, such as Hydretain and LESCO Moisture Manager). Therefore, they are eventually broken down by soil microbial activity. Research and users have demonstrated that the most effective hygroscopic humectants products have been able to reduce water use by up to 50% and will typically perform for up to 90 days. In addition to providing general conservation of water, hygroscopic humectants aid in seed germination, transplant establishment and in establishing sod and sprigs. Hygroscopic humectants have also been used to suppress dust on baseball infields, horse arenas, dirt race tracks, etc.
Super absorbant polymers. This technology tracks its orgin to a patent by Monsanto in 1963. They described polymers as “strings of large molecules that chemists use like Tinker Toys, adding, subtracting or linking them together to create diverse uses ranging from filling for disposable diapers to dental products. Baby diapers are a well-known use for this technology.
Polymers have been adapted for use in soil to improve water availability to plants. They are used to “increase a soil’s water holding capacity, increase pore sizes and numbers in the soil, increase germination rates, and decrease or mitigate the effect of soil compaction on plant growth. The five main types of soil polymers available commercially include:
- Cross-linked polyacrylamides (gel forming)
- Non-cross-linked polyacrylamides (water soluble)
- Polyacrylates
- Polyacrylontrile
- Starch-grafted copolymers
The most commonly used polymer is the cross-linked polyacrylamide. Soil polymers occur in a crystalline form. When exposed to water, they expand into a gelatin-like block. When used in soils, they function as mini-reservoirs of water. They absorb water and hold it until the plant removes the water. The literature indicates that cross-linked polyacrylamide polymers used in the field will absorb and hold 80 to 200 times their weight in water or more. Their ability to hold soil water is influenced by the amount of polymer in the soil; the type of polymer used and soil characteristics, such as salt content. As the concentration of ions increases in water, the amount of hydration by the polymer decreases. The lifespan of polymers is thought to range from 2 to 10 years, depending on the type of polymer and soil conditions.
The literature reports that the time between irrigation events can be extended with the use of polymers, but the actual water savings with use of these products is dependent on application rates and soil conditions. Cost of these products may be a limiting factor for effective application rates.
Initially, polymers were used to help manage water in potted plants, ornamental beds and in planting trees and shrubs. Over the years, soil-applied polymer use has expanded to turf applications. They are used in the establishment of sod and sprigs, improving seed germination and in general turf use. The challenge in using polymers on established turfgrass is delivering the polymer crystal to the rootzone. Some turf managers will aerate the turf and drag the crystal into the holes. In addition to this practice, there are now machines that will inject or “plant” the polymer crystals into the soil.
Surfactants/wetting agents. Surfactants or wetting agents are probably the most common products used to manage soil moisture. These materials are used for a number of applications in turf and plant management, including relief from localized dry spots, improved drainage, assist the efficiency of various pesticides, reduced dew and frost accumulation, improved seed germination, reduced fairy ring damage, alliviation of soil compaction, improved irrigation efficiency, diminished dust on dirt paths, enhanced firmness of golf course bunker sand and more.
Surfactants stand for SURFace ACTive AgeNTS (SURFACTANTS). These are agents that affect the surface of a liquid or solid. As previously stated, the formation of waxy, non-polar coatings on soil particles is the cause of hydrophobic conditions. The non-polar soil particle surface will not attract, and may actually repel, the polar water molecule, which prevents irrigation water or rainfall from infiltrating soils to hydrate plants. Creating a polar surface allows water molecules to enter and fill the soil. The surfactant has a non-polar and a polar end on the molecule. The non-polar end of the surfactant molecule aligns with the non-polar surface of the organic soil coating, leaving the polar end exposed outward from the soil particle. This allows the polar water molecules to be attracted to the polar surfactant molecules therefore overcoming the hydrophobic condition.
There are many different kinds of surfactants, most of which fall into these four basic categories:
- Anionic – Form negatively-charged ions in water
- Cationic – Form positively-charged ions in water
- Nonionic – Does not ionize in water
- Amphoteric – Take on the ionization of the water
Non-ionic surfactants are the most common products used in the turf industry due to their safety, compatibility with other products and ease of use. As technology has improved, a number of catetories of non-ionic surfactants have been developed. These include:
- Polyoxyethylene (POE). This is older technology originally developed to treat localized dry spots. They can be phytotoxic.
- Block Co-Polymer Surfactants. These are the most commonly used turfgrass surfactants. They are safer and are effective in treating soil water repellency, improving soil water content and plant available water. This category has two sub-categories: Straight Block Co-Polymers and Reverse Co-Polymers.
- Alkyl Polyglucoside Surfactants. These are made from sugar molecules reacted with a fatty acid and are considered as naturally derived. When blended with a block co-polymer the performance appears to be better than either technology alone. These blended technologies appear to increase water infiltration, improve water availability and enhance irrigation efficiencies.
- Modified Methyl Capped Block Co-Polymer. This is a class of surfactant that is a modification of the co-polymer class. This technology forms a thinner, more continuous film around the soil particle.
- Humic Substance Redistribution Molecules. These molecules allow water penetration through the soil profile by disrupting the hydrophobic supramolecular humic association, most prevalent in the top one to two centimeters on the soil, which lead to localized dry spots.
- Multi-branched Regenerating Wetting Agents. Most surfactants have linear molecules. These products have a much higher molecular weight and multiple branched molecules. Each branch essentially functions as wetting agent itself.
Surfactants/wetting agents have been demonstrated to possess many functions in the management of water in and around turfgrass and other plant systems. When discussing the maximization of water use efficiencies, these products tackle the barriers (non-polar coatings in the soil) that prevent water from moving into and distributing throughout the soil. Research has shown that surfactants/wetting agents can significantly improve soil moisture content and reduce variability in soil water content, improving soil moisture uniformity. In addition, they have been shown to “reduce localized dry spot incidence, allow for longer periods between irrigation events, and reduce hand watering in isolated areas.”
Surfactants/wetting agents are available in liquid and granular forms. The amount of water conserved, longevity of the product and cost may vary based on product type and local conditions.
There are a wide variety of technologies available to help manage and conserve water. As with pesticide selection, the key to success is to identify the cause(s) of water challenges. If salts or bicarbonates are a problem, there are calcium and acid based treatments. If non-polar soil particle coatings are the challenge, there are a variety of surfactant/wetting agent solutions for this condition. If poor water holding capacity is the issue, there are hygroscopic humectants and polymers.
As a final note, it is advisable to remember to not think linearly. Often, there is not one single issue with one single solution. The best solution for the management and conservation of water may be to combine technologies. A very common example of this is the combination of hygroscopic humectants with surfactants technologies. In this situation, the surfactant will allow water with the hygroscopic humectant to enter and disperse throughout the soil where hydrophobic non-polar organic coatings exist. Water can uniformly disperse throughout the rootzone. Then, the hygroscopic humectant can reduce evaporative loss for maximum plant water use.
Thinking outside the box and using all tools available gives turf managers the ability to maximize water use efficiency and optimize turf and plant performance.
Top photo: Hydrated vs. dry polymers
Article written by James Spindler, CPAg, CCA, CPSS. He is director of agronomy at Ecologel Solutions, LLC; president of BioPro Technologies, LLC; and research director for the OJ Noer Foundation.